Abstract
Background: clofarabine (CLO) is a nucleoside analogue with efficacy in acute myeloid and lymphoblastic leukemia (ALL). We evaluated whether CLO added to induction and consolidation would improve event free survival (EFS) in adult patients.
Methods: patients, aged 18-70 years, with newly diagnosed precursor B- or T-ALL were eligible and randomized for standard treatment or combined with CLO. Standard treatment for younger (18-40 years) patients consisted of a pediatric inspired schedule (HOVON70, Rijneveld et al. 2011) and older patients (41-70 years) were treated with a semi-intensive schedule (HOVON71, Daenen et al. 2012). Patients with Ph positive ALL received imatinib during treatment, but not in combination with CLO. Patients were stratified according to age and immunophenotype (B- vs T-ALL). CLO (5 days, 30 mg/m2) was administered during 2 courses, i.e. during prephase with prednisone and as a single drug during a second consolidation course. The CLO dose was established during a run-in phase and was approved by the DSMB. High risk disease was defined as high WBC count at diagnosis, no CR after induction or high risk cytogenetic/molecular abnormalities (Ph or BCR-ABL positivity, MLL aberrations, hypodiploidy and complex karyotype). Allogeneic stem cell transplantation (alloSCT) with an HLA identical sibling donor was offered as consolidation to all patients and in patients with high-risk ALL a sibling or alternative donor was used. Primary endpoint was EFS. Secondary endpoints included CR, MRD measured by RQ-PCR of rearranged Ig or TCR genes, disease free (DFS) and overall survival (OS), toxicities and treatment related mortality. This trial was supported by Sanofi Genzyme and registered at www.trialregister.nl as NTR2004.
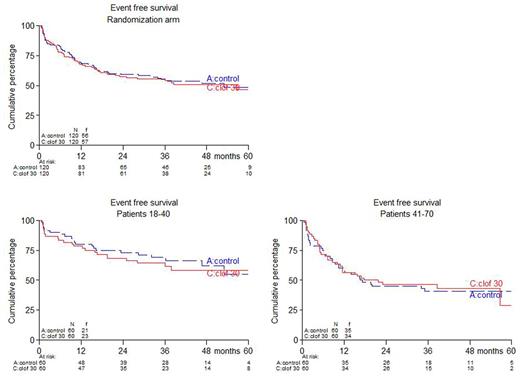
Results: between December 2010 and November 2016, 340 patients were randomized. Here we report results of the first 120 younger and 120 older patients, i.e. 240 patients in total. No differences between study arms were observed with respect to pretreatment characteristics including age (median 41 year), risk group, Ph positivity, WHO-PS and immunophenotype. Respectively, 80% and 73% of patients ≤40 years completed protocol treatment according to arms A (control) and B (CLO); these percentages were 67% and 62% for patients >40 years. Estimated EFS was 49% and 46% at 5 years from randomization for arm A and B resp. (hazard ratio (HR): 1.05, 95% confidence interval (CI): 0.72-1.51, p=0.81; adjusted for age and phenotype). For patients ≤40 years, 5-year EFS was 58% vs 55% in arm A vs B, while in patients > 40 years EFS was 41% vs 29% in arm A vs B. CR rate was 87% and 88 % in arm A vs B and in both age groups. MRD was assessed at multiple time points during treatment and available for 117 (48%) patients, 58 and 58 in arm A and B resp. MRD response was defined by levels <10-4. 37/58 patients (64%) obtained MRD after consolidation 1 in arm A vs 49/59 (83%) in arm B (p=0.022). 49 (41%) of patients proceeded to alloSCT in arm A vs 54 (45%) in arm B (p=0.60). After a median follow up of 40 months (range: 1-72), 5-year OS was 59% in arm A vs 51% in arm B (HR = 1.17, 95% CI: 0.78-1.76, p=0.45). In patients who reached CR, 5-year DFS was 56% in arm A and 53% in arm B (HR = 1.08, 95% CI: 0.70-1.66, p=0.74), while 5-year relapse rates were 27% and 25% (HR = 1.03, 95% CI: 0.58-1.81, p=0.92), and 5-year non-relapse mortality was 17% vs 22 % (HR = 1.15, 95% CI: 0.59-2.25, p=0.69). No significant differences with respect to EFS, OS, and DFS emerged after evaluation with censoring of alloSCT recipients. Serious adverse events (SAEs) were slightly more often observed in CLO treated patients: 71% vs 82% in ≤40y (p=0.28) and 54% vs 65% in >40y (p=0.27). More infections grade 3-4 were observed in CLO treated patients: 44 vs 64% in arm A and B resp. (p=0.003).
Conclusions: while complete evaluation of all 340 randomized patients is still ongoing, analysis of the first 240 randomized patients with sufficient follow-up (med 40 months) shows that addition of CLO is feasible. Toxicities included a non-significant increase of SAEs and a significant increase of infections. So far, a non-significant lower number of patients receiving CLO fully completed protocol treatment. Despite a higher MRD response in the CLO arm EFS, DFS and OS were not improved so far. Longer follow-up and analysis of all patients is required to determine whether a better MRD response translates into better outcome.
Rijneveld: Pfizer: Membership on an entity's Board of Directors or advisory committees; Jazz pharmaceuticals: Research Funding. Petersen: Sanofi: Membership on an entity's Board of Directors or advisory committees. Selleslag: Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Schipperus: Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal